72-year old Henry Povinelli is an active volunteer. He devotes around 30 hours a week to AARP and has lived a healthy lifestyle since he was young. After surviving colon cancer in 2017, he was again diagnosed with cancer in November 2019 — this time sarcoma, a rare type of bone and soft tissue cancer.
“My sarcoma diagnosis wasn’t a shock, but it was a huge disappointment. However, I was very fortunate. I was in active surveillance mode and my care team was able to detect it early,” says Henry.
After going through a round of chemotherapy, Henry qualified for the ongoing sarcoma clinical trial at Levine Cancer Institute (LCI). This trial, funded by the Paula Takacs Foundation for Sarcoma Research (PTF), gives a unique opportunity to investigate new, more effective front line treatments for sarcoma. Clinical trials often focus on more mainstream cancers, but the PTF has uniquely positioned itself to improve treatments for rare sarcoma cancers, funding clinical trials and other research taking place at Levine Cancer Institute and Levine Children’s, their sole beneficiaries.
Susan Udelson, Executive Director of the Paula Takacs Foundation, was thrilled that the foundation funded this clinical trial for pediatric and adult sarcoma patients. When asked why the PTF is well-positioned to benefit sarcoma cancer research locally, she responded by saying:
“The Paula Takacs Foundation is quite unlike the handful of other sarcoma organizations in the country because it focuses on funding local sarcoma research for all types of pediatric and adult sarcomas,” said Udelson. When the founder, Paula Takacs, was diagnosed with sarcoma in 2004 it was before Levine Cancer Institute was conceptualized. Therefore, she had to travel outside of the Carolinas in order to access cutting-edge clinical trials. It was grueling on her and on her family. When LCI opened its doors in 2012, Paula and the PTF Board decided to direct 100 percent of fundraising efforts to help develop an outstanding local sarcoma research program in Charlotte.
“With this first LCI investigator initiated, clinical trial fully enrolling 30 patients and achieving really promising results that were presented at an international meeting, it literally brings our mission to life by showing that we empowered the local sarcoma community through investing in research, which is expanding global hope,” said Udelson, “After all, breakthroughs in this disease space can come from any institution worldwide – where outstanding and innovative research is happening – including our own backyard.”
The Sarcoma Study
Thanks to their partnership with the Paula Takacs Foundation, Levine Cancer Institute has developed a homegrown, fully funded local trial designed to improve treatments and outcomes for patients with sarcomas. Because of the trial’s promising results, it was selected among thousands of other cancer trials for a prestigious “poster discussion” at ASCO’s (American Society of Clinical Oncology) annual meeting at the end of May 2020 — the world’s largest oncology meeting. It was also the first virtual ASCO annual conference due to COVID-19.
Currently, there’s only one “mainstream” first-line treatment for all soft tissue sarcomas. It was approved by the U.S. Food & Drug Administration (FDA) in 1974. There are more than 50 types of soft tissue sarcoma, so one generalized treatment is like shooting a single arrow at a broad target.
This is one of the many reasons why LCI is proud to be at the forefront of groundbreaking research for rare cancers. In fact, it’s one of few cancer centers starting research devoted to rare cancers.
Every year, the Paula Takacs Foundation presents LCI with a significant annual gift, totaling about $1.1 million to date, to help fund the many research projects being conducted.
“Clinical trial participants and their families feel a critical spark of hope, and we are so proud to help them achieve that,” says Udelson.
“Hope is a very powerful force. Every single patient deserves it, including those diagnosed with exceedingly rare cancers like sarcomas. We work tirelessly to make that a possibility.”
Henry’s Promising Progress
Henry currently has a soft tissue growth on his liver and in the lower lobe of his left lung. Since these places are difficult to operate on, an effective non-surgical treatment is vital for management and recovery. This study focuses on the use of Pembrolizumab, a treatment that is typically more gentle on patients than sarcoma’s mainstream treatment, which involves chemotherapy.
When Henry gets his regular checkups at Levine Cancer Institute, Edward Kim, MD, FACP, FASCO, is there to check on his progress. Kim is the chairman for the solid tumor oncology program at LCI and helps to lead this study and collect data regarding the effectiveness of the trial. Also, Michael B. Livingston, MD works tirelessly as the principal investigator of the trial. He enrolls all the patients and works with oversight from Dr. Kim.
“There's certain organic research that we do that starts here at Levine Cancer Institute that we develop, we lead, and then we are able to report. One of those this year is a clinical trial that we have developed in sarcoma,” says Dr. Kim. “You don't find many clinical studies out there in sarcoma so in collaboration with PTF, we came up with the idea to study immunotherapy in patients with soft tissue sarcoma.”
When Henry was treated for his colon cancer in 2017, his scans revealed he was cancer-free following his surgery. When he was diagnosed with sarcoma, he went through chemotherapy, which had difficult side effects. Then, he qualified for the sarcoma clinical trial offered at LCI.
“I’m fortunate to have no side effects with the medication I am receiving on this clinical trial. This treatment is less toxic too. I get treatment in 3-week intervals at an infusion center connected to LCI. As long as we see positive results, we’re going to continue,” says Henry.
But the benefits don’t just involve innovative care. At the infusion center, Henry gets to meet nurses and staff that he now calls friends.
“I’ve been able to develop that social connection with that team while I’m there getting treatment for cancer. They’re very social and professional in a real positive way. The care team is very focused on my success and responsive to me to the point where I feel like I’m the only person in that center being treated — even though I am not.”
Progress Takes the Mainstage
Every 5 to 6 weeks Henry gets a CT scan at Levine Cancer Institute to check his progress. So far, all the results have indicated tumor shrinkage and no new growth.
When Henry found out that LCI’s clinical trial for sarcoma was chosen to be prominently featured in the world’s premiere oncology conference, he replied, “I initially didn’t realize how much I was at the forefront. It’s very exciting; I love to be a part of a real success story in the way the treatment is working. I’ve had a lot of positive experiences that I attribute to the clinical trial.”
Analyzing The Data
Dr. Kim, who actively works with the study, gives us a summary of the study’s progress:
“The results were very impressive for a small, but single-center, pilot study. We saw response rates, meaning shrinkages of tumors, close to 40%, and disease control in the 80% range. There were some side effects and toxicities as Doxorubicin, the chemotherapy, does have some side effects, but they were overall manageable. And we were very encouraged by this data.”
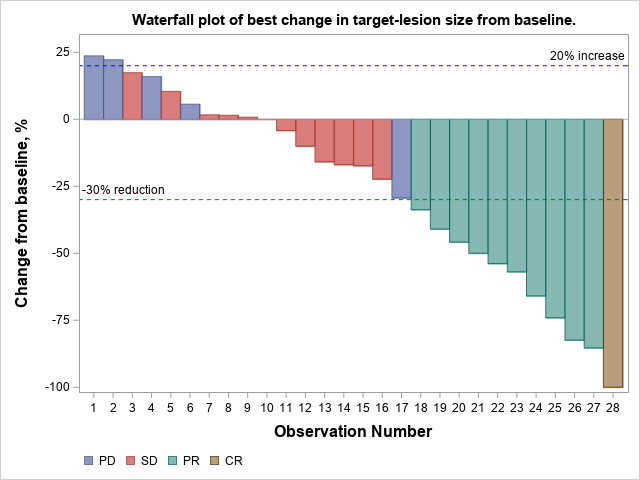
Figure 1: Waterfall Plot of Best Change in Target-Lesion Size from Baseline. In 3 subjects, disease progression was due to the presence of new lesions and not by an increase in the size of the target lesion from baseline. Post-baseline scans were not available for 2 subjects due to death prior to the second set of scans.
In this waterfall plot, each patient’s best response to pembrolizumab + doxorubicin (the study regimen) is demonstrated by a single bar. Every patient in the trial received the regimen, the majority of whom had shrinkage of their tumor. One patient had complete disappearance of their cancer.
“I'm proud of everything that's been accomplished. I think the Charlotte region, the Carolina region, both North and South, should also be proud to have such an institute like Levine Cancer Institute, and we're going to continue to push forward and drive the science even further delivering really top-notch clinical care as well as research excellence,” says Dr. Kim.
Today, Henry carries the same positive attitude that led him to beat cancer the first time.
“Currently I’m feeling really good, I’m feeling healthy, I am excited to KEEP GOING. I advise others going through this to let the treatments work. It won’t be easy but don’t give up. At the same time, the investment in yourself is worth it because you’ll get back the things you enjoy doing. Cancer may change how I do things, but I'm still going to do things. I’m not going to do less and it’s not going to stop me.”
To donate to the PTF, click here.
To donate to fund additional local trials, go to the Atrium Health Foundation.