Hypertrophic Cardiomyopathy Center of Excellence
Sanger Heart & Vascular Institute’s nationally recognized hypertrophic cardiomyopathy (HCM) program provides state-of-the-art care and has participated in key clinical trials designed to evaluate novel therapies for this condition.
Recent Highlights
Introducing a Novel Myosin Inhibitor
Sanger is participating in the CAMZYOS® (Mavacamten) Risk Evaluation and Mitigation Strategy (REMS) program. This program encompasses REMS for the rollout of a new cardiac myosin inhibitor for patients with obstructive HCM. Our participation is a coordinated effort involving MD and APP prescribers as well as a Sanger pharmacist and clinical social worker.
Awards and Recognition
Awards and Recognition
-
1 of 46
in the Country
Hypertrophic Cardiomyopathy Association -
1 of 2
in North Carolina
Hypertrophic Cardiomyopathy Association
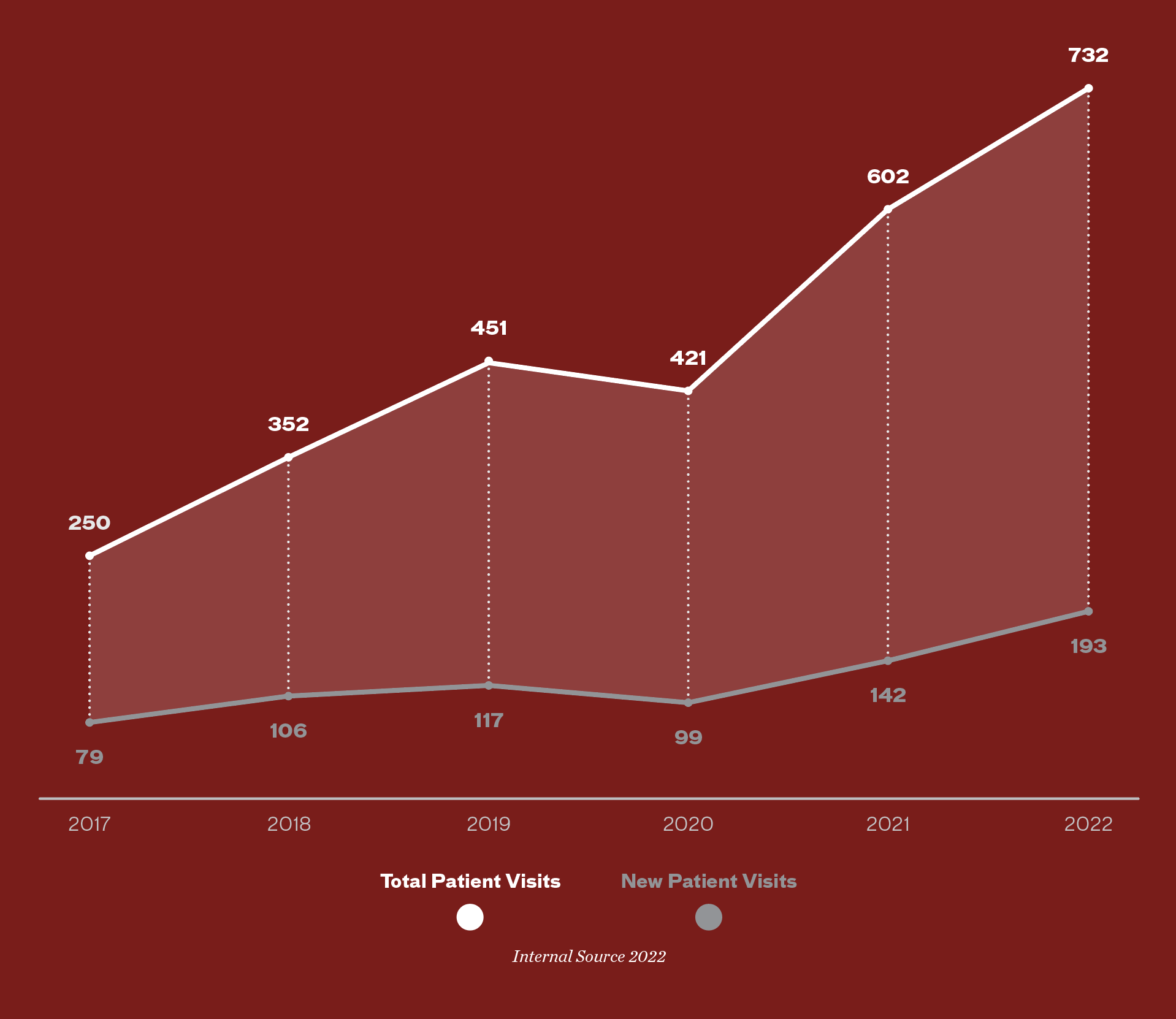
Hypertrophic Cardiomyopathy Patient Volumes
-
200
New Hypertrophic Cardiomyopathy Patients
2022
Internal Data, YTD September 2022 Annualized -
532
Returning Hypertrophic Cardiomyopathy Patients
2022
Internal Data, YTD September 2022 Annualized -
150
Hypertrophic Cardiomyopathy Genetic Counseling Visits
Internal Data, 2022
Growth of Hypertrophic Cardiomyopathy Program
Research and Clinical Trials
Bristol-Myers-Squibb Study MYK-461-007 (MAVE-LTE): Long-term extension study of mavacamten in patients completing the MYK-461-005 and MYK-461-006 clinical investigations
Bristol-Myers-Squibb Observational Study CV027-012 (DISCOVER-HCM): A prospective registry study to assess real-world patient characteristics, treatment patterns and longitudinal outcomes in patients receiving mavacamten and other treatments for symptomatic obstructive HCM
Cytokinetics Clinical Study CY-6022: Open-label extension study to collect long-term safety and tolerability data for CK-3773274, including MRI sub-study REDWOOD-OLE
Imbria Pharmaceuticals Study (IMPROVE-HCM): A study to evaluate the safety, tolerability and efficacy of IMB-1018972 in patients with non-obstructive HCM
Bristol-Myers-Squibb Study CV027-031 (ODYSSEY-HCM): A randomized, double-blind, placebo-controlled clinical study to evaluate mavacamten in adults with symptomatic non-obstructive HCM
Cytokinetics Clinical Study CY-6031 (SEQUOIA-HCM): A phase III, multicenter, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of IP in adults with symptomatic obstructive HCM and left ventricular outflow tract obstruction
Cytokinetics Clinical Study CY-6021 (REDWOOD-HCM): Randomized evaluation of dosing with CK-3773274 in obstructive outflow disease in HCM
- Cohort 3: Evaluation of CK3773274 in obstructive HCM in patients being treated with disopyramide
- Cohort 4: Evaluation of CK3773274 in patients with symptomatic non-obstructive HCM
Publications and Presentations
Recommendations For Multimodality Cardiovascular Imaging of Patients with Hypertrophic Cardiomyopathy: An Update from the American Society of Echocardiography, In Collaboration with the American Society of Nuclear Cardiology, The Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. Nagueh, SF, Phelan DM, et al. J Am Soc Echocardiogr 2022; 35:533-69. PMID: 35659037
Post-systolic shortening index by echocardiography evaluation of dyssynchrony in the non-dilated and hypertrophied left ventricle. Saijo Y, Wang TKM, Chan N, Sperry BW, Phelan DM, Desai MY, Griffin B, Grimm RA, Popovic ZB. PLoS One. 2022 Aug 25;17(8): e0273419.
Research letter: Aficamten as add-on therapy in medical treatment-refractory obstructive hypertrophic cardiomyopathy (REDWOOD-HCM, cohort 3 analysis). Owens AT, Masri A, Abraham T, Choudhury L, Radar F, Symanski JD, Turer A, Wong T, Tower-Radar A, Coats C, Fifer M, Olivotto I, Solomon S, Watkins H, Robertson L, Meng L, Paige SL, Wohtman A, Kupfer S, Malik FI, Heitner SB, Maron MS. Circulation (in submission).
Practical approach to echocardiographic imaging in patients with hypertrophic cardiomyopathy. Mitchell CC, Phelan DM, Symanski JD, Frye C, et al. J Am Soc Echocardiogr (in submission).
Case Presentation: An Unusual Case of Left Ventricular Hypertrophy and Heart Failure. Presented by John Symanski, MD. 2022 South Atlantic Cardiovascular Society. July 17, 2022.
Hypertrophic Cardiomyopathy – presentation session moderated by Dermot M. Phelan, MD and Neal Kon, MD. 2022 South Atlantic Cardiovascular Society.
Amyloid, HCM, Fabry’s? How to tell. Dermot M. Phelan, MD. American College of Cardiology 2022 Scientific Session. April 2, 2022.
Presenters at Hypertrophic Cardiomyopathy Association “Bighearted Warriors Unite” live webinar, which highlighted Sanger. Sept. 15, 2022.