Research and Clinical Trials
Sanger Heart & Vascular Institute continues to pioneer industry-sponsored and investigator-led studies. Our physicians are currently leading research in every area of cardiovascular services.
Overview
Overview
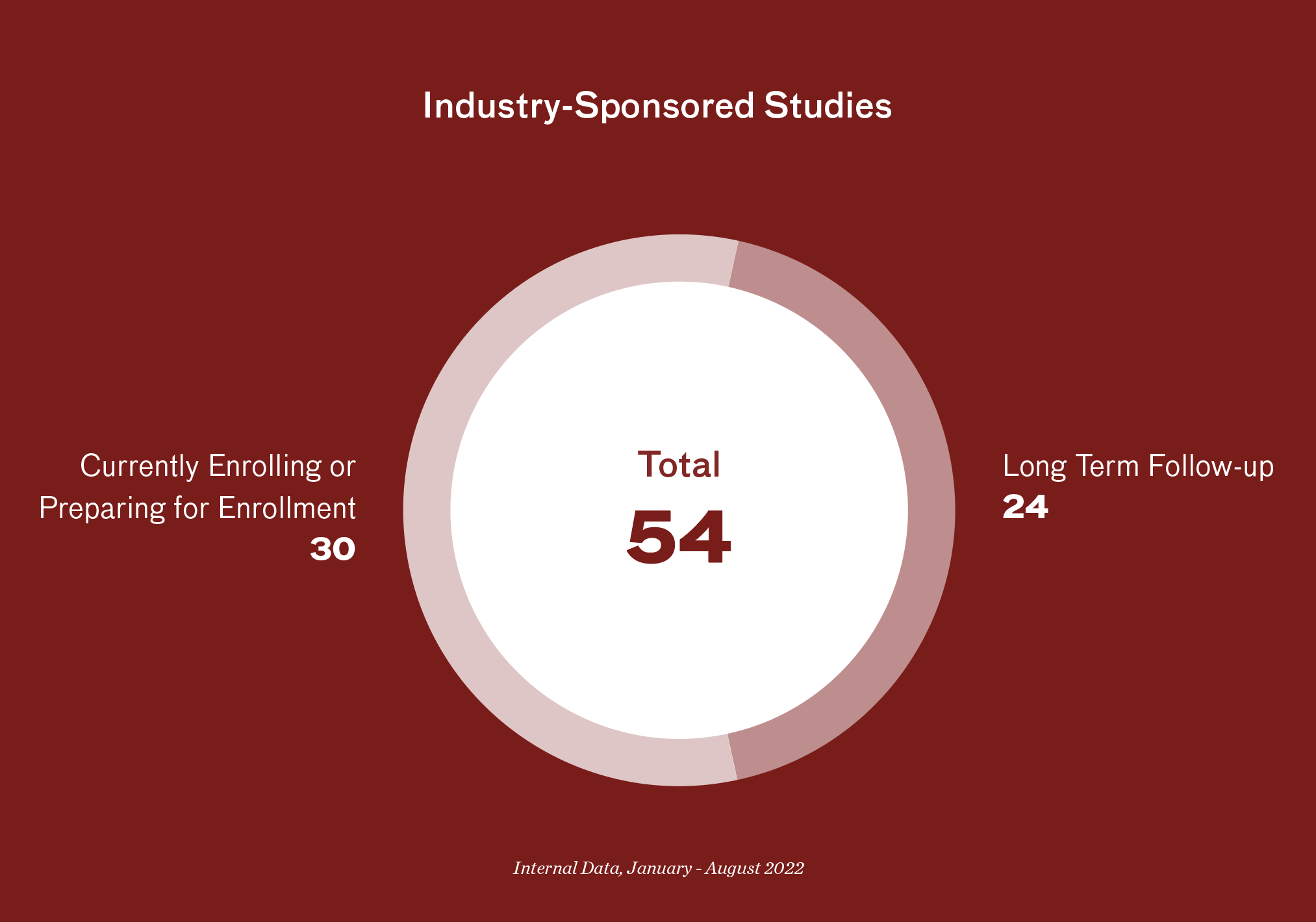
- 51 industry-sponsored clinical trials actively enrolling or in long-term follow-up
- 20+ industry-sponsored studies in the startup phase
- 66 investigator-led studies/registries
- 37 published papers
- 7 abstracts/poster presentations at national meetings
-
Read more about our research and search our clinical trials.
Recent Highlights
A Sanger team successfully implanted the Cephea transcatheter mitral valve replacement system in June 2022. This was only the fourth human implant of this system; Sanger was the second center in the world to perform the procedure.
A Sanger physician was part of a collaboration that yielded three NIH grants:
- Repurposing of Metformin for Older Patients with Heart Failure with Preserved Ejection Fraction (MET-PEF), a three-year project with a requested budget of $1.5 million. This will be a clinical trial with sites at Sanger and Atrium Health Wake Forest Baptist. The study is based on the joint Atrium Health – Wake Forest Clinical and Translational Science Institute pilot grant awarded in 2021.
- Atrium Health Wake Forest Baptist – Atrium Health HeartShare Clinical Center. This is a pivotal development because the National Heart, Lung and Blood Institute plans for the HeartShare project to be its sole, major investment in the area of HFpEF for at least the next five years. The HeartShare project will total $50 million across all sites.
- REHAB-HFpEF trial. This is a phase III trial involving 20 sites, 46 investigators, staff and 880 patients, with $38 million in funding across all sites.
A Sanger team is leading a study titled Evaluation of Novel Artificial Intelligence Software in the Automated Quantification of Echocardiographic Measurements.
Objectives include:
- Evaluating the software’s efficiency in quantitative echocardiographic measurements compared to standard manual measurements.
- Assessing whether variability between automated measurements and manual measurements between observers can be used to predict bias in different facilities.
- Evaluating whether the software reduces bias and variability of measurements between observers compared to manual evaluation.
We are the coordinating center for a multicenter lead extraction study with four additional sites nationwide. The goal is to establish a prospective multicenter lead extraction registry that includes a diverse, representative cohort of sites that will provide insights and guidance into best practices for transvenous lead extraction and improve patient outcomes.
Our team is part of the Comparison of Methods of Pulmonary Blood Flow Augmentation in Neonates: Shunt Versus Stent (COMPASS) trial. This study is the first of its kind to randomize neonates with ductal-dependent pulmonary blood flow to either ductal artery stent or surgical systemic to pulmonary artery shunt, and to perform an intention-to-treat analysis of their outcomes. This is an important opportunity for our physicians to answer a key question for patients with congenital heart defects, and Sanger is among a select few centers that have both interventional and surgical teams that are strong enough to participate in this study.
Publications and Presentations
Electronically Monitored Corticosteroid Eye Drop Adherence after Trabeculectomy Compared to Surgical Success. McGlumphy EJ, Dosto NO, Johnson TV, Quigley HA. Ophthalmol Glaucoma. 2022 Jan 4.
Effect of Treatment with Sacubitril/Valsartan in Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. Mann DL, Givertz MM, Vader JM, Starling RC, Shah P, McNulty SE, Anstrom KJ, Margulies KB, Kiernan MS, Mahr C, Gupta D, Redfield MM, Lala A, Lewis GD, DeVore AD, Desvigne-Nickens P, Hernandez AF, Braunwald E. LIFE Investigators. JAMA Cardiol. 2022 Jan 1.
The First Sulfate-Pillared Hybrid Ultramicroporous Material, SOFOUR-1-Zn, and Its Acetylene Capture Properties. Sensharma D, O’Hearn DJ, Koochaki A, Bezrukov AA, Kumar N, Wilson BH, Vandichel M, Zaworotko MJ. Angew Chem Int Ed Engl. 2021 Dec 20.
Case 298: Skeletal Fluorosis Secondary to Huffing. Mayer MJ, Gliedt JA, Shaker JA, Symanski JS. Radiology. 2022 Feb.
When to Consider Cardiac MRI in the Evaluation of the Competitive Athlete After SARS-CoV-2 Infection. Phelan D, Kim JH, Drezner JA, Elliott MD, Martinez MW, Chung EH, Krishan S, Levine BD, Baggish AL. Br J Sports Med. 2022 Jan 27.
Demographics, Clinical Interests, and Ophthalmology Skills Confidence of Medical Student Volunteers and Non-volunteers in an Extracurricular Community Vision Screening Service-learning Program. Burton E, Assi L, Vongsachang H, Swenor BK, Srikumaran D, Woreta FA, Johnson TV. BMC Med Educ. 2022 Mar 4.
SARS-CoV-2 Active Infection Prevalence and Seroprevalence in the Adult Population of St. Louis County. Goss CW, Maricque BB, Anwuri VV, Cohen RE, Donaldson K, Johnson KJ, Powderly WG, Schechtman KB, Schmidt S, Thompson JJ, Trolard AM, Wang J, Geng E. Ann Epidemiol. 2022 Mar 8.
Transcarotid Artery Revascularization (TCAR) in the Frail. Yammine H, Briggs C, Arko FRA 3rd. Ann Vasc Surg. 2022 Mar 4.
Long-Term Clinical Impact of Contrast-Associated Acute Kidney Injury Following PCI: An ADAPT-DES Substudy. Mohebi R, Karimi Galougahi K, Garcia JJ, Horst J, Ben-Yehuda O, Radhakrishnan J, Chertow GM, Jeremias A, Cohen DJ, Cohen DJ, Maehara A, Mintz GS, Chen S, Redfors B, Leon MB, Stuckey TD, Rinaldi MJ, Weisz G, Witzenbichler B, Kirtane AJ, Mehran R, Dangas GD, Stone GW, Ali ZA. JACC Cardiovasc Interv. 2022 Mar 3.
Characterization of Cerebral Embolic Capture Using the SENTINEL Device During Transcatheter Aortic Valve Implantation in Low to Intermediate-Risk Patients: The SENTINEL-LIR Study. Kawakami R, Gada H, Rinaldi MJ, Nazif TM, Leon MB, Kapadia S, Krishnaswamy A, Sakamoto A, Sato Y, Mori M, Kawai K, Cornelissen A, Park JE, Ghosh SKB, Abebe BG, Romero M, Virmani R, Finn AV. Circ Cardiovasc Interv. 2022 Mar 11.
Identifying Key Components of a Web-Based Long Term Care Planning Intervention For Family Caregivers of Individuals With Intellectual/Developmental Disabilities. Chicas VE, Steinway C, Chen J, Schwartz MC, Wright C, Cornacchia M, Davis TW, Berens JC, Riddle I, Woodward JF, Jan S. J Appl Res Intellect Disabil. 2022 Mar 11.
Single-Center Experience with Paraquat Exposure in Nine Patients. McKinzie BP, Powell BD, Sljivic S, Hollowell J, Maxwell E, Nizamani R, King B, Williams FN. J Burn Care Res. 2022 Mar 23.
Subjective Assessment Underestimates Surgical Risk: On the Potential Benefits of Cardiopulmonary Exercise Testing for Open Thoracoabdominal Repair. Bailey DM, Halligan CL, Davies RG, Funnell A, Appadurai IR, Rose GA, Rimmer L, Jubouri M, Coselli JS, Williams IM, Bashir M. J Card Surg. 2022 Apr 29.
Assessment of the Impact of the COVID-19 Pandemic On Health Services Use. Johnson KJ, Goss CW, Thompson JJ, Trolard AM, Maricque BB, Anwuri V, Cohen R, Donaldson K, Geng E. Public Health Pract (Oxf). 2022 Jun 3. Epub 2022 Apr 5.
Dual Antiplatelet Therapy Discontinuation, Platelet Reactivity, and Adverse Outcomes After Successful Percutaneous Coronary Intervention. Redfors B, Kirtane AJ, Liu M, Musikantow DR, Witzenbichler B, Rinaldi MJ, Metzger DC, Weisz G, Stuckey TD, Brodie BR, Ben-Yehuda O, Mehran R, Stone GW. JACC Cardiovasc Interv. 2022 Apr 25.
Outcomes and Revenue Generation of a Community-based Screening at a Center in the United States: The SToP Glaucoma Program. Varadara V, Wahl M, Gajwani P, David J, Dutson M, Zhao D, Guallar E, Swenor BK, Johnson TV, Friedman DS. SToP Glaucoma Study Group. J Glaucoma. 2022 Apr 6.
Controversies in Enhanced Recovery After Cardiac Surgery. Shaw AD, Guinn NR, Brown JK, Arora RC, Lobdell KW, Grant MC, Gan TJ, Engelman DT. Perioperative Quality Initiative (POQI) and Enhanced Recovery after Surgery–Cardiac investigators. Perioper Med (Lond). 2022 Apr 28.
‘Fit for Surgery’: The Relationship Between Cardiorespiratory Fitness and Postoperative Outcomes. Rose GA, Davies RG, Appadurai IR, Williams IM, Bashir M, Berg RMG, Poole DC, Bailey DM. Exp Physiol. 2022 May 17.
Atrial Cannulation During Resuscitative Clamshell Thoracotomy. Willis G, Robinson JN, Green JM, Dieffenbaugher ST, Madjarov JM, LeNoir BJ, Frederick JR, Sing RF, Cunningham KW. Am Surg. 2022 May 15.
Current Landscape of Produce Prescription Programs in the US. Newman T, Lee JS, Thompson JJ, Rajbhandari-Thapa J. J Nutr Educ Behav. 2022 Jun.
Twist Angle Tuning of Moiré Exciton Polaritons in van der Waals Heterostructures. Fitzgerald JM, Thompson JJP, Malic E. Nano Lett. 2022 May 20.
Retina-sparing suprachoroidal intraocular foreign body resulting in cyclodialysis cleft. Kane CP, Johnson TV, Sachdeva MM. Am J Ophthalmol Case Rep. 2022 May 3.
Analyses of Transplanted Human Retinal Ganglion Cell Morphology and Localization in Murine Organotypic Retinal Explant Culture. Zhang KY, Johnson TV. STAR Protoc. 2022 Apr 18.
Atrial Cannulation During Resuscitative Clamshell Thoracotomy. Willis G, Robinson JN, Green JM, Dieffenbaugher ST, Madjarov JM, LeNoir BJ, Frederick JR, Sing RF, Cunningham KW. Am Surg. 2022 May 15.
External Wrapping of Ascending Aortic Dissection with Intramural Hematoma. Madjarov JM, Katz MG, Fazal S, Simionescu DT. J Thorac Cardiovasc Surg. 2022 Jul.
US for Traumatic Nerve Injury, Entrapment Neuropathy, and Imaging-guided Perineural Injection. Symanski JS, Ross AB, Davis KW, Brunner MC, Lee KS. Radiographics. 2022 Jul 1.
Myocarditis in the Athlete: A Focus on COVID-19 Sequelae. Symanski JD, Tso JV, Phelan DM, Kim JH. Clin Sports Med. 2022 Jul. Epub 2022 Feb 17.
Nasal and Parotid Blood Pool Activity Is Significantly Correlated with Metabolic Syndrome Components and Sleep Apnea. Phillips WT, Issa NJ, Elhalwagi SB, Draeger HT, Schwartz JG, Gelfond JA. Metab Syndr Relat Disord. 2022 Jun 22.
Aquaporin 4 is Not Present In Normal Porcine and Human Lamina Cribrosa. Kimball EC, Quillen S, Pease ME, Keuthan C, Nagalingam A, Zack DJ, Johnson TV, Quigley HA. PLoS One. 2022 Jun 16.
Single-Cell Transcriptome and Cell Type-Specific Molecular Pathways of Human Non-Alcoholic Steatohepatitis. Fred RG, Steen Pedersen J, Thompson JJ, Lee J, Timshel PN, Stender S, Opseth Rygg M, Gluud LL, Bjerregaard Kristiansen V, Bendtsen F, Hansen T, Pers TH. Sci Rep. 2022 Aug 5.
Extensive Co-Binding and Rapid Redistribution of NANOG and GATA6 During Emergence of Divergent Lineages. Thompson JJ, Lee DJ, Mitra A, Frail S, Dale RK, Rocha PP. Nat Commun. 2022 Jul 23.
An Epigenome Atlas of Neural Progenitors Within the Embryonic Mouse Forebrain. Rhodes CT, Thompson JJ, Mitra A, Asokumar D, Lee DR, Lee DJ, Zhang Y, Jason E, Dale RK, Rocha PP, Petros TJ. Nat Commun. 2022 Jul 20.
Unilateral Perforant Path Transection Does Not Alter Lateral Entorhinal Cortical or Hippocampal CA3 Arc Expression. Cooper TL, Thompson JJ, Turner SM, Watson C, Lubke KN, Logan CN, Maurer AP, Burke SN. Front Syst Neurosci. 2022 Jun 30.
Enhanced Excitonic Features In An Anisotropic ReS2/WSe2 Heterostructure. Usman A, Adel Aly M, Masenda H, Thompson JJP, Gunasekera SM, Mucha-Kruczyński M, Brem S, Malic E, Koch M. Nanoscale. 2022 Aug 4.
Association Between Open Payments-Reported Industry Transfers of Value and Prostaglandin Analog Prescribing in the US. Nguyen AM, Anderson KE, Anderson G, Johnson TV. JAMA Ophthalmol. 2022 Jul 28.
Restoring Partial Vision To a Blind Patient. Benowitz LI, Dowling JE, Giger RJ, Johnson TV, Zack DJ. Fac Rev. 2022 Jun 27.